JUN 2020 | Report Format: Electronic (PDF)
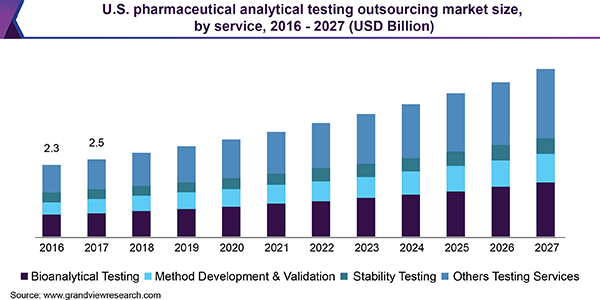
The global pharmaceutical analytical testing outsourcing market size is expected to reach USD 11.4 billion by 2027, according to a new report by Grand View Research, Inc., registering a CAGR of 8.3% over the forecast period. Increasing pipelines for biological candidates, rising demand for additional analytical details on drugs, and process development by regulatory agencies are boosting the market growth.
Analytical testing helps in providing real-time product quality control along with optimizing and monitoring processes, characterizing biosimilars and biologics, and improving productivity. One of the main reasons for outsourcing analytical testing services is to benefit from skilled and experienced professionals, as well as to access expensive analytical equipment which are not available in-house.
Increased development of biologics is also creating a demand for analytical testing services. In the last three decades, biologics have taken the center stage in the discovery and development of drugs. Moreover, there is a high demand to define higher-order biological structures. The need for better product characterization, along with comparability studies, especially for biosimilars, further leads to drug companies outsourcing these services.
A patent cliff, particularly for small molecules in the pharmaceutical industry leads to a decline in revenue, thereby affecting profits. Over the next few years, several best-selling biologics would come toward the end of patent life, such as Humira. Drug innovators are therefore under constant pressure to bring new products through the pipeline at a faster rate. Developing advanced analytical testing to assess and monitor the quality attributes of these products requires a broader set of equipment and expertise, which is beyond the internal capacity of pharma companies. This would subsequently lead to increased instances of outsourcing pharmaceutical analytical testing services.
To request a sample copy or view summary of this report, click the link below:
www.grandviewresearch.com/industry-analysis/pharmaceutical-analytical-testing-outsourcing-market
Further key findings from the report suggest:
- The outsourcing of clinical bioanalytical testing services emerged as the largest segment in 2019 with a share of around 79% owing to the increasing number of clinical trials
- The extractable and leachable services segment is expected to witness a significant growth rate owing to an increasing number of vendors offering these services at competitive prices
- North America dominated the global market with a share of 54.2% in 2019 as the region is one of the top manufacturing hubs of highly reliable, complex, and high-end pharmaceuticals
- Some of the players operating in the pharmaceutical analytical testing outsourcing market are Toxikon, Inc.; Intertek Group PLC; SGS SA; Eurofins Scientific; Boston Analytical; Pace Analytical Services, LLC; West Pharmaceutical Services, Inc.; Intertek Group Plc; Pharmaceutical Product Development, LLC; WuXi AppTec, Inc.; and Charles River Laboratories International, Inc.