Rare Disease Genetic Testing Market Growth & Trends
The global rare disease genetic testing market size is expected to reach USD 2.52 billion by 2030, registering a CAGR of 13.94% over the forecast period, according to a new report by Grand View Research, Inc. Effective regulatory plans to combat rare disease is one of the key drivers of the industry. Furthermore, the presence of a substantial number of registries that provide data and relevant information about related diseases has aided in revenue growth over the past years. Ongoing conferences to raise awareness about rare and ultra-rare conditions are anticipated to boost the adoption of diagnostic kits and services. For instance, Ergomed and PSR Orphan Experts, with their offices in the U.K., Germany, the Netherlands, Poland, and other countries, participate in various activities that are aimed at raising awareness in this area.
Moreover, the Canadian Organization for Rare Disorders (CORD) offers a strong platform to streamline health policy and a healthcare system that is dedicated to the management of patients with disorders. The agency works with clinicians, researchers, governments, and the diagnostic industry to advance R&D, diagnosis, treatment, and service availability for all rare conditions in the country. As per the National Institutes of Health (NIH), around 30 million Americans have been identified with one of 7,000+ known rare diseases. The number of patients undergoing disease testing is expected to increase in the coming years with growing awareness. The U.S. celebrates Rare Disease Day and promotes developments in this area by raising awareness.
In addition, the presence of the Rare Diseases Clinical Research Network (RDCRN), an NIH-funded research network of 23 active consortia or research groups that includes patients, researchers, and clinicians who are focused on the diagnosis & treatment of disorders is anticipated to positively impact the industry. Amidst the COVID-19 pandemic, patients with undiagnosed and rare diseases have been facing significant health challenges. According to a study published in January 2021 by a group of researchers from the U.S., there is an urgent need for the development of approaches that can reduce the serious challenges affecting Rare & Undiagnosed diseases (RUD) patients and families. The challenges include diagnostic and/or prognostic uncertainty coupled with medical complexity leading to poor health outcomes.
In response to the challenges created by the pandemic, patient support groups and professional societies are actively involved in addressing these challenges. For instance, the Rare Chromosome Support Group (Unique), the British Society for Genomic Medicine, and Genetic Alliance U.K. are actively involved in supporting, advocating, and providing information to patients with diseases. Several rare conditions remain unclassified. The charity SWAN U.K. estimated that around 6,000 children are born each year with genetic conditions in the U.K. As per the agency, the conditions are extremely rare and cannot be named. Thus, there is a lack of proper diagnosis and prognosis for evidence-based treatment. At present, around 50% of the children who undergo genetic testing in the U.K. would not receive a confirmed diagnosis.
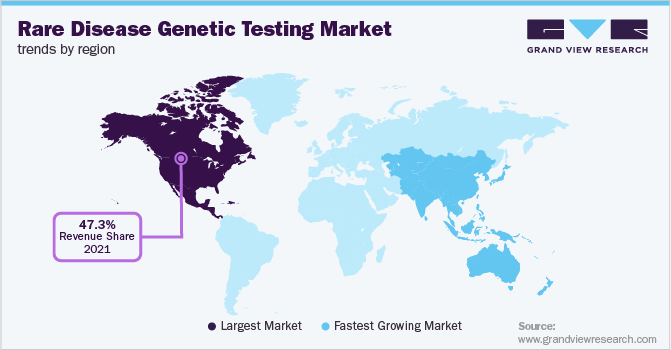
Around 50% of the children with learning disabilities and approximately 60% of children with congenital conditions do not receive a definitive diagnosis to identify the cause of their disabilities. Furthermore, the lack of awareness among patients and families about diagnosis and genetic testing has further impeded the industry’s growth. North America dominated the industry in 2021 due to the high incidence of rare diseases, a large number of registries, the presence of substantial numbers of R&D facilities in this area, and extensive investments in diagnosis. Asia Pacific is expected to register the fastest CAGR during the forecast years owing to the presence of a substantial number of organizations that are focusing on disease management.
Request a free sample copy or view the report summary:
Rare Disease Genetic Testing Market Report
Rare Disease Genetic Testing Market Report Highlights
- The immunological disorders segment held a significant revue share in 2021. A rise in the cases of multiple sclerosis drives the growth as is one of the key focus areas in this segment
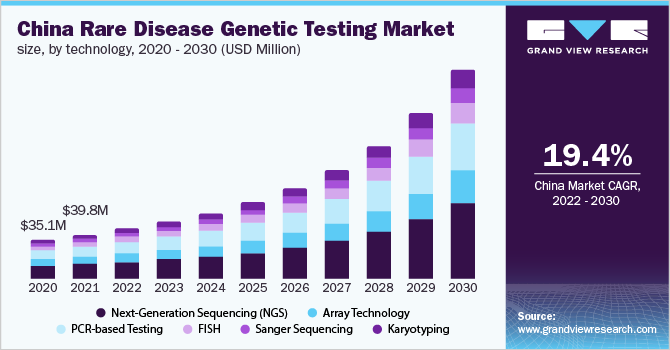
- Based on technology, the NGS segment is expected to register the fastest CAGR from 2022 to 2030
- This is due to the availability & adoption of NGS-based gene panels for cancer, neurologic disease, CVD, pediatric conditions, psychiatric disorders, and another rare disease testing
- The molecular genetic tests specialty segment held the largest revenue share in 2021 due to the high adoption in research & clinical settings. A rise in CLIA-waived molecular genetic tests also offers lucrative growth opportunities
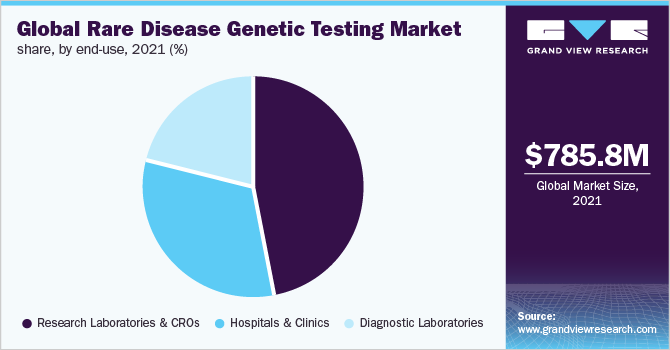
- The research laboratories & CROs end-user segment led the industry in 2021 owing to a large number of genetic tests conducted in research laboratories and CROs
List of Key Players in the Rare Disease Genetic Testing Market
- Quest Diagnostics Inc.
- Centogene N.V.
- Invitae Corp.
- 3billion, Inc.
- Arup Laboratories
- Eurofins Scientific
- Strand Life Sciences
- Ambry Genetics
- Perkin Elmer, Inc.
- Realm IDX, Inc.
- Macrogen, Inc.
- Baylor Genetics
- Color Genomics, Inc.
- Health Network Laboratories
- PreventionGenetics
- Progenity, Inc.
- Coopersurgical, Inc.
- Fulgent Genetics Inc.
- Myriad Genetics, Inc.
- Laboratory Corporation of America Holdings
- Opko Health, Inc.
- Artemis DNA