North America And Europe Preclinical Medical Device Testing Service Market Growth & Trends
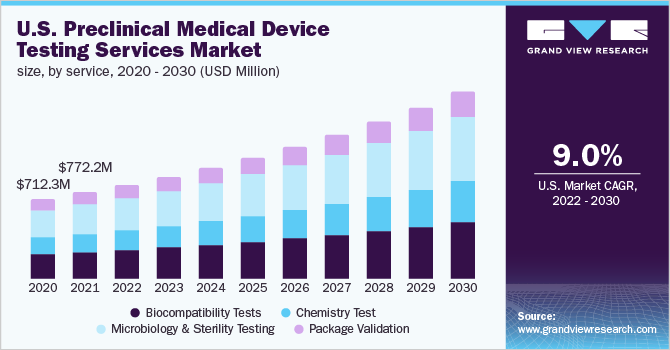
The North America and Europe preclinical medical device testing services market size is expected to reach USD 2.92 billion by 2030, expanding at a CAGR of 8.7%, according to a new report by Grand View Research, Inc. The increase in the number of small medical devices lacking in-house testing capabilities and complexity in product design are the major factors driving the growth of the market.
There has been an increase in the number of players operating in the market over the last decade. Due to this large number, it has witnessed fierce competition. To sustain the market, a mix of defensive and offensive marketing strategies is used. For instance, extensive R&D, competitive pricing, new product launches, collaborative development, regional expansion, and mergers and acquisitions.
The COVID-19 pandemic has created a huge demand for these services. The rise was not significant in the first half, but it became more significant in the second as the industry adapted to operating during the pandemic. There has been an increase in the production and testing of personal protective equipment, and several projects that were put on hold because of COVID have resumed personal protective equipment. The epidemic has increased demand for a wide range of medical gadgets, diverting attention from those needed for surgery. COVID-19 vaccinations, ventilators, and pulse oximeters are the main goods seeing an increase in demand.
Request a free sample copy or view the report summary:
North America And Europe Preclinical Medical Device Testing Services Market Report
North America And Europe Preclinical Medical Device Testing Services Market Highlights
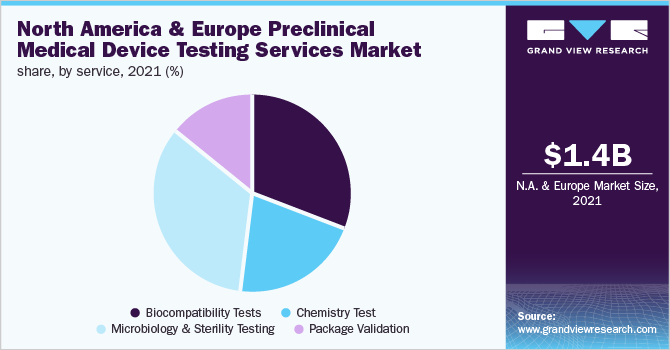
- Microbiology & Sterility Testing segment dominated the market with a revenue share of 34.3% in 2021. It is one of the major tests included in pre-clinical medical device testing. These examinations aid in identifying any microorganisms that could be present in the apparatus
- Based on test type, sterility test and validation led the market with a revenue share of 45.7% in 2021
- North America led the global market in 2021 and is projected to witness the fastest CAGR of 8.8% during the forecast years
- In Europe, the market held a considerable revenue share of 36.6% in 2021. This is due to the rising demand for cost-cutting and increasing complexity in product designing, which is supporting the growth of the region
List of Key Players of North America And Europe Preclinical Medical Device Testing Services Market
- SGS SA
- Toxikon, Inc.
- Eurofins Scientific
- Pace Analytics Services LLC
- WUXI AppTec
- North America Science Associates, INC
- TÜV SÜD AG
- American Preclinical Services
- Sterigenics International LLC
- Charles River Laboratories International, Inc
- Nelson Labs