May 2020 | Report Format: Electronic (PDF)
The global transdermal drug delivery system market is expected to reach USD 81.4 billion by 2024, according to a new report by Grand View Research, Inc. The unprecedented global shift in the adoption of unhealthy lifestyles is presumed to be responsible for the high prevalence of chronic diseases, such as cancer and diabetes, which is expected to drive the clinical urgency to incorporate transdermal drug delivery systems in the future treatments. Moreover, the rising geriatric population base, which is highly susceptible to developing the aforementioned chronic diseases are expected to propel the demand for highly efficacious pharmacological drugs.
The market is driven by technological advancements in transdermal drug delivery devices, which is anticipated to serve the market with future growth opportunities. These advancements include advanced, third-generation, transdermal drug delivery technologies including iontophoresis, ultrasound, and microneedles or mechanical arrays. These technologies serve as effective transdermal drug diffusion alternatives that are capable of improving overall patient health and quality of life. For instance, the advent of matrix-controlled transdermal drug diffusion comprising a drug reservoir and a thermo effector, enhances skin permeation and enables controlled drug delivery.
To request a sample copy or view summary of this report, click the link below:
www.grandviewresearch.com/industry-analysis/transdermal-drug-delivery-systems-industry
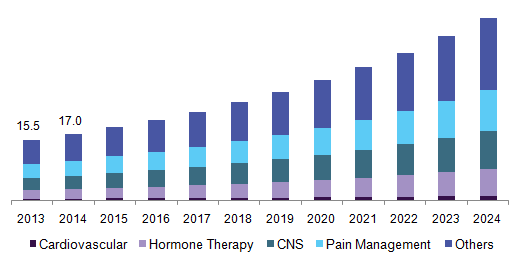
Further key findings from the study suggest:
- The cardiovascular application segment is expected to exhibit growth at a significant CAGR of over 12.0% during the forecast period. This is a consequence of the high clinical urgency to curb hypertension and the need for precise drug delivery at a constant rate, which has led to the development of transdermal drug delivery systems.
- The mechanical array in the technology segment is anticipated to witness an exponential CAGR of over 12.5%. The lucrative growth is attributed to the availability of different forms of microneedle transdermal systems, such as solid, hollow, and dissolving microneedles, as per the suitability and needs of the patients.
- In 2015, North America dominated the overall Transdermal Drug Delivery System Market at over 50.0%. The consistent number of product launches being undertaken and the continual participation of the prominent players in conducting clinical trials are anticipated to promote the incorporation of transdermal drug delivery systems.
- The key players are increasingly involved in initiating collaborative strategies and frequent product launches to capture a larger market share. For instance, in September 2014, Noven Pharmaceuticals, Inc. received the FDA approval for Minivelle, estradiol transdermal system to be used for the prevention of postmenopausal osteoporosis. Furthermore, in June 2015, Boehringer Ingelheim GmbH sponsored the clinical trials, which investigated the bioequivalence of transdermal clonidine administration.