May 2020 | Report Format: Electronic (PDF)
The global pneumatic nebulizers market size was valued at USD 490.6 million in 2015 and is expected to reach a value of USD 893.9 million by 2025. The expansion is attributed to the increasing spread of infection of COVID-19, which has turned into a pandemic along with the growing geriatric population base. The introduction of small, efficient and portable new systems featuring aerosol drug delivery technology with minimal drug wastage is expected to drive the market growth. For example, in October 2018, Philips launched InnoSpire Go, its smallest and lightest portable hand-held nebulizer. This innovative technology not only provides portable, fast and effective medication delivery but is also proven to reduce treatment time by up to 25 percent.
To request a free sample copy report, click the link below:
www.grandviewresearch.com/industry-analysis/pneumatic-nebulizer-market
Generally, nebulizer is termed used for an apparatus, which can turn liquids into a fine mist. To accomplish this, nebulizers use gas flows at a high speed. These nebulizers are used in paint spray cans and most dominantly in asthma inhalers. Also, it is recently found out that nebulizers are more effective and easier to use than metred-dose inhalers (MDIs) with spacers. Pneumatic nebulizers are a specific type of nebulizers which are used as a drug delivery device, which is a godsend in the case of diseases such as:
· Asthma
· Chronic Bronchitis
· Cystic Fibrosis
· Chronic Obstructive Pulmonary Diseases(COPD)
· COVID-19
· Other respiratory Diseases
Pneumatic nebulizers accept the medicine in liquid form, using oxygen, compressed air or ultrasonic power to break up solutions and suspensions into small aerosol droplets that are inhaled from the mouthpiece of the device. The reason these pharmaceuticals are inhaled instead of ingested is in order to target their effect to the respiratory tract, which speeds onset of action of the medicine and reduces side effects, compared to other alternative intake routes.
Further key findings from the report suggest:
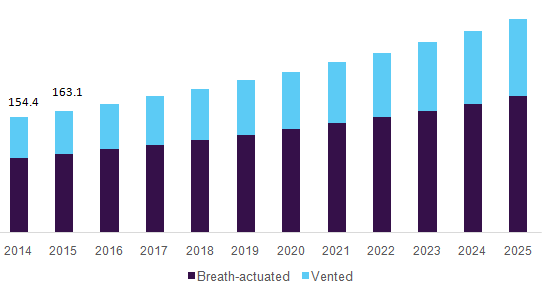
- Vented nebulizers dominated the product type segment in 2015 owing to their high availability and affordable cost. Moreover, industry contributors are introducing new solutions that enable patient data access in one single platform. In November 2015, CareFusion introduced a new respiratory solution at the American Association for Respiratory Care Congress.
- North America is estimated to dominate the pneumatic nebulizers market in 2015. The introduction of advanced technological devices and the supportive medical coverage offered in this region is expected to further fuel market growth. For instance, in March 2015, Sunovion Pharmaceuticals, Inc. announced the initiation of Phase III clinical trial for its SUN-101 solution using PARI Pharma’s eFlow nebulizer system for COPD.
- Asia Pacific is the fastest growing market owing to its huge aging population base, increasing focus on preventive care, and government initiatives promoting technological innovations. In July 2015, Tribeca Care launched its online healthcare store to supply homecare respiratory products, wherein prominent medical brands such as Philips, Omron, Nidek, Karma, ResMed, and Vissco were brought under one roof.
- Sector players are integrating healthcare with technology to improve upon the current patient monitoring devices in the vertical for respiratory diseases. Moreover, increasing awareness around pre-assessment devices has further accelerated the demand for pneumatic nebulizers. In June 2016, Philips introduced the Patient Adherence Management Service at the 2016 SLEEP event to better patient compliance to sleep therapy.
Major Industry Participants Include:
· Omron Corporation
· Philips
· PARI Pharma
· GE Healthcare
· Allied Healthcare
· CareFusion Corporation
· Becton, Dickinson and Company
· Agilent Technologies
· Medline Industries, Inc.
· Briggs Healthcare
Some of the major developments that have taken place over the past few years include:
· OMRON Healthcare, a global leader in medical equipment for home health monitoring and therapy, announced the acquisition of privately-held 3A Health Care s.r.l., a leader in the development and production of specialized aerosol therapy devices and surgical aspirators.
· Zambon, a privately held long-established Italian multinational company, extends its presence in severe respiratory diseases through the acquisition of Breath Therapeutics, a biopharmaceutical company developing an innovative inhalation therapy for Bronchiolitis Obliterans Syndrome (BOS), a rare fatal respiratory disease currently in phase III. Breath Therapeutics’ drug device platform consists of a proprietary liposomal cyclosporine A for inhaled drug delivery with eFlowⓇ nebulizer technology from PARI Pharma.
· U.S. medical equipment supplier Becton Dickinson and Co acquired C R Bard Inc, in a $24 billion cash-and-stock deal, adding Bard’s devices to its portfolio in the high-growth sectors of oncology and surgery.