The drug device combination market is
expected to reach USD 177.7 billion by 2024, according to a new study by Grand
View Research, Inc. The drug device combination market is primarily driven by
exponential growth in the production of advanced drug delivery devices coupled
with the growing chronic disease burden, which is anticipated to be one of the
primary factors to accelerate the overall demand of the market throughout the
forecast period. The unprecedented adoption rate of these products is also
believed to be a consequence of its inherent benefits over the traditional
pharmacological alternatives to deliver therapeutics, which in turn influences
pharmaceutical, biotechnology, and medical device companies to work toward
further developing these products for subsequent commercialization.
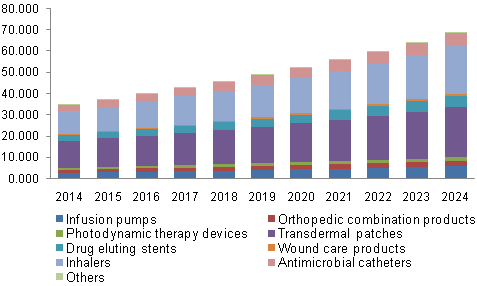
North America Drug Device Combination Market, By Product,
2014 - 2024 (USD Billion)

As
a consequence of the high patient and physician demand, it is observed that regulatory
agencies have developed specific competencies and regulations for combination
product manufacturers. The regulatory healthcare agencies across the globe,
notably the Pharmaceuticals Medical Devices Agency, Japan and the U.S. FDA,
require manufacturers to gain deep insights pertaining to specific patient
requirements in numerous countries to ensure accurate and timely market access
to the drug device combination products. In addition, these regulatory bodies
ensure the development of sophisticated combination products for enhanced drug
delivery with a high safety level. Such heightening sophistication in the
overall manufacturing process is believed to accentuate the demand for
combination products and encourage high participation by the pharmaceutical and
medical device companies.
To view summary of this report, click the link
below:
www.grandviewresearch.com/industry-analysis/drug-device-combination-market
www.grandviewresearch.com/industry-analysis/drug-device-combination-market
Further Key Findings From the Study Suggest:
· Segment with transdermal patches held the dominant
share of over 35.0% in the product segment in 2015. The substantial share can
be attributed to the consistent technological advancements that present this
drug delivery mode with benefits over conventional treatment methods.
· Inclusion of third-generation iontophoresis and
microneedle technology, matrix-controlled transdermal systems, and thermo
effectors in transdermal patches are some of technological innovations
contributing toward the segment share
· Inhalers are anticipated to grow at a profitable
rate throughout the forecast period owing to the rising prevalence of asthma
and COPD coupled with the surging awareness of innovative therapies including
controlled or programmable drug dosing
· Thenebulizers are expected to grow at a lucrative
rate during the forecast period owing to easy drug administration with active
pharmaceutical ingredients delivered in the form of fine mist. This facilitates
direct delivery of therapeutics to the lungs, which proves to be the most
convenient way of providing medication to infants and toddlers, thereby
propelling the demand for nebulizers during the forecast period.
· In 2015, North America dominated the regional drug
device combination vertical with a share of over 42.0%. The dominant share is a
consequence of high investment in the R&D sector of advanced drug delivery
technologies, the resultant technological advances in this space, and the
subsequent high adoption of innovative combination products owing to convincing
therapeutic outcomes.
· Asia Pacific is expected to witness growth at an
exponential CAGR during the forecast period. The exponential growth rate is
attributed to the increasing healthcare spending, improving infrastructure
quality pertaining to drug development and discovery, increasing outsourcing
manufacturing activities, and the adoption of compact and low cost techniques.
· The key players are consistently employing new
product development and collaborative strategies to attain a higher share and
gain a competitive advantage in the sector. For instance, in August 2016, W.L.
Gore & Associates, Inc. announced the FDA approval of its dual-component
stent, GORE TIGRIS vascular stent with a fluoropolymer/nitinol design.
· The aforementioned product launch was carried out
to expand options for the treatment of peripheral artery disease, which allows
easy insertion and better clinical outcomes, even in a challenging anatomy
View more reports of this category by Grand View
Research at:
Grand View
Research has segmented the drug device combination market on the basis of
product and region:
Global Drug Device Combination Product Outlook
(Revenue, USD Billion, 2013 - 2024)
·
Infusion Pumps
o Volumetric
o Disposables
o Syringes
o Ambulatory
o Implantables
o Insulin
·
Orthopedic Combination Products
o Bone
Graft Implants
o Antibiotic
Bone Cement
·
Photodynamic Therapy Devices
·
Transdermal Patches
·
Drug Eluting Stents
o Coronary
stents
o Peripheral
vascular stents
·
Wound Care Products
·
Inhalers
o Dry
powder
o Nebulizers
o Metered
dose
·
Antimicrobial Catheters
o Urological
o Cardiovascular
o Others
·
Others
Drug Device Combination Regional Outlook (Revenue,
USD Billion, 2013 - 2024)
·
North America
o U.S.
o Canada
·
Europe
o UK
o Germany
·
Asia Pacific
o Japan
o China
o India
·
Latin America
o Mexico
o Brazil
·
MEA
o South
Africa
View press release of this research report by Grand View Research:
www.grandviewresearch.com/press-release/global-drug-device-combination-market
www.grandviewresearch.com/press-release/global-drug-device-combination-market
About Grand View Research
Grand View
Research, Inc. is a U.S. based market research and consulting company,
registered in the State of California and headquartered in San Francisco.
The company provides syndicated research reports, customized research
reports, and consulting services. To help clients make informed business
decisions, we offer market intelligence studies ensuring relevant and
fact-based research across a range of industries, from technology to chemicals,
materials and healthcare.
For more
information: www.grandviewresearch.com